The Importance of Laser Marking in the Medical Manufacturing Industry
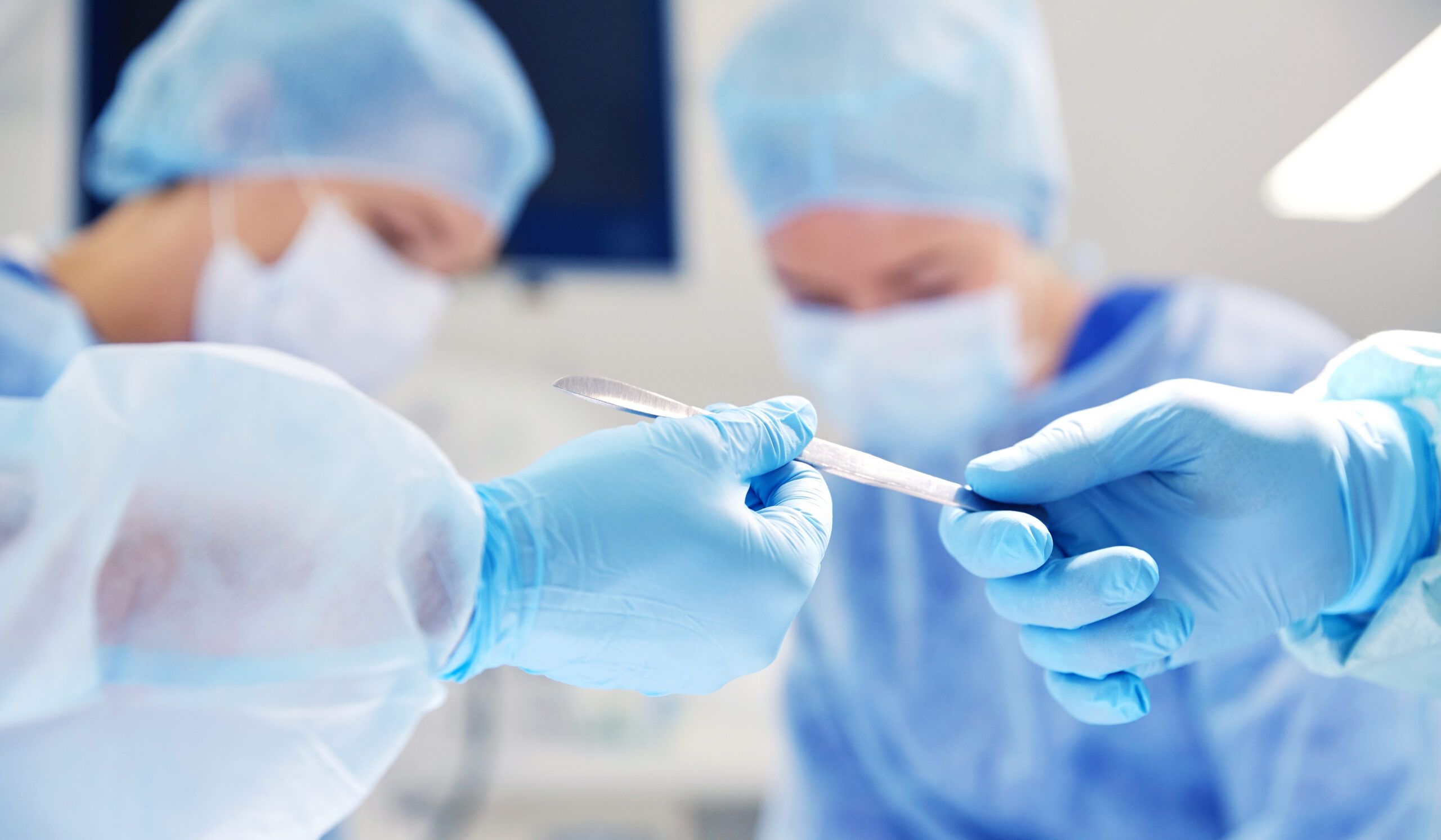
The medical industry demands the highest standards of safety, precision, and traceability. World Health Organization defines a medical device as any instrument or apparatus intended by the manufacturer for medical purposes. Medical devices are critical in common and complex medical procedures, from bandaging a sprained ankle to performing disc replacement surgery.
Whether it’s a surgical tool, an implant, or diagnostic instruments, each device must be reliably identified, tracked, and rendered safe for repeated sterilization and use. Traditional labeling methods, like ink printing, adhesive labels, or stamping, often fall short when faced with sterilization, wear, or the need for fine detail.
Laser marking is the preferred method for ensuring reliable, permanent, and compliant marking of medical devices. In this blog, we’ll explore why laser marking excels in medical applications, highlight critical benefits, and show how Beamer’s technology aligns with regulatory requirements and manufacturing needs.

Why Medical Devices Demand Permanent, High-Quality Marking
Traceability & Regulatory Compliance
Most medical devices carry unique device identifiers (UDI) to ensure every instrument can be traced through its full lifecycle. The U.S. Food and Drug Administration mandates clear and consistent marking of medical devices through the UDI system. This fosters patient safety, streamlines post-market surveillance, and drives innovation within the medical device industry. By using laser marking, manufacturers ensure compliance of the medical industry with regulatory standards, while creating machine- and human-readable codes that last over the lifetime of the device.
Safety, Cleanliness, and Biocompatibility
Medical devices demand sterility and chemical safety. Labels, inks, or adhesives introduce foreign substances that may compromise hygiene or biocompatibility. Laser marking avoids these issues entirely because it’s a non-contact process that uses no inks or consumables, thus no risk of contamination. Additionally, laser marking minimizes mechanical stress or surface damage that might occur with stamping or engraving, preserving the underlying material.

Key Benefits of Laser Marking Over Traditional Methods
When it comes to precision, laser marking has no equal. The laser light is highly concentrated and focused into an incredibly small beam which scans the surface in a desired pattern. This allows laser marking technology to create intricate and complex designs that are difficult to achieve with mechanical tools. The following are the key distinctive laser marking features:
- Durability: Marks stay legible after repeated sterilization, chemical exposure, and wear & tear.
- Traceability: Permanent, high-contrast marks (serial numbers, barcodes, 2D data matrix codes, lot/batch numbers) enable traceability.
- Precision: Fine detail, small characters, or micro-marking on implants and small components is achievable, preserving device functionality.
- Clean, Non-Contact Process: No inks, no adhesives, and no chemicals, making the process clean-room compatible.
Laser marking also works across the full spectrum of medical tools and devices. Some examples include:
- Surgical instruments (scalpels, forceps, clamps, drill bits)
- Medical implants (bone plates, dental implants, orthopedic prostheses)
- Catheters, medical tubing, connectors, small plastic or polymer components
- Diagnostic and lab devices (syringes, test-tubes, pipettes)
Lastly, laser marking is a sound choice for sustainability-oriented businesses. The ecological footprint of traditional marking solutions remains unresolved, as the ongoing need for mill bits, ink, chemicals, and other consumables continues to accumulate waste.
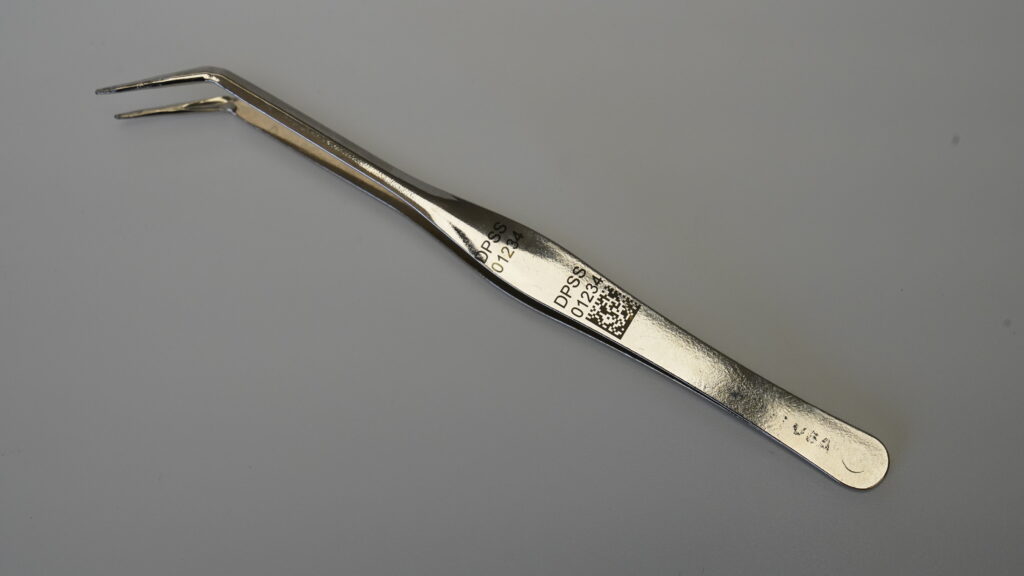
The Role of Laser Marking in Quality Control
By having permanent, legible identifiers, manufacturers and hospitals can track usage of each instrument, which helps with time maintenance, replacements, or reordering more efficiently. In the case of a recall or product issue, these UDIs enable quick identification of affected instruments, thus reducing risk, enabling targeted recalls, and protecting patient safety.
Traceability also helps prevent counterfeiting, which can be a serious concern in medical devices, where counterfeit or substandard products can endanger patients. In short, laser marking supports not only manufacturing but also long-term quality assurance, regulatory compliance, and operational safety.
Why Beamer Laser Marking Systems is Positioned to Lead
Given all these needs and benefits, there’s strong demand for reliable, flexible, and compliant laser marking solutions in the medical device industry. Here’s where Beamer can add value:
- Customization & Flexibility
- Compliance-Ready Markings
- Scalability & Automation
- Long-Term Value for OEMs and Hospitals
Beamer Laser Marking Systems provides medical device manufacturers with marking solutions that increase productivity, efficiency, and meet or exceed regulatory requirements. And with an unmatched 100,000+ hour lifespan, our American-made, industrial grade fiber lasers provide consistent performance over extended periods of use, and reduced downtime and maintenance costs.

Beamer Laser Marking Systems isn’t just about creating clear and compliant markings on your medical devices; it’s about streamlining your entire production process. The speed and precision of laser marking enable you to streamline tedious marking processes, allowing for greater operational efficiency in your production lines. This efficient, single-step process eliminates the need for multiple marking stages, saving you valuable time and resources. By investing in Beamer’s laser marking technology, you can achieve significant cost savings and experience a smoother, more efficient production flow.
Invest in Beamer’s laser marking technology today and achieve significant cost savings! Contact us before the end of 2025 to learn how you can deduct up to $1.5 million in qualifying equipment purchased and placed in service with section 179!

